What is the atomic number and mass number of an atom when a β (beta) particle is emitted?
Posted in Atoms, Molecules and Nuclei | Email This Post
|
Email This Post
|
An unstable nucleus contains excess of neutrons, and this causes neutron transforming into proton by emitting an electron.
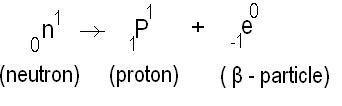
When a β particle is emitted by an atom, its atomic number increases by 1 and mass number doesn’t change.

Rutherford and Soddy’s law of β decay states that “whenever an β particle is emitted from a radioactive nucleus, the atomic number decreases by 1 and the mass number remains the same and the position of the element shifts by one place to right in the periodic table”.
